
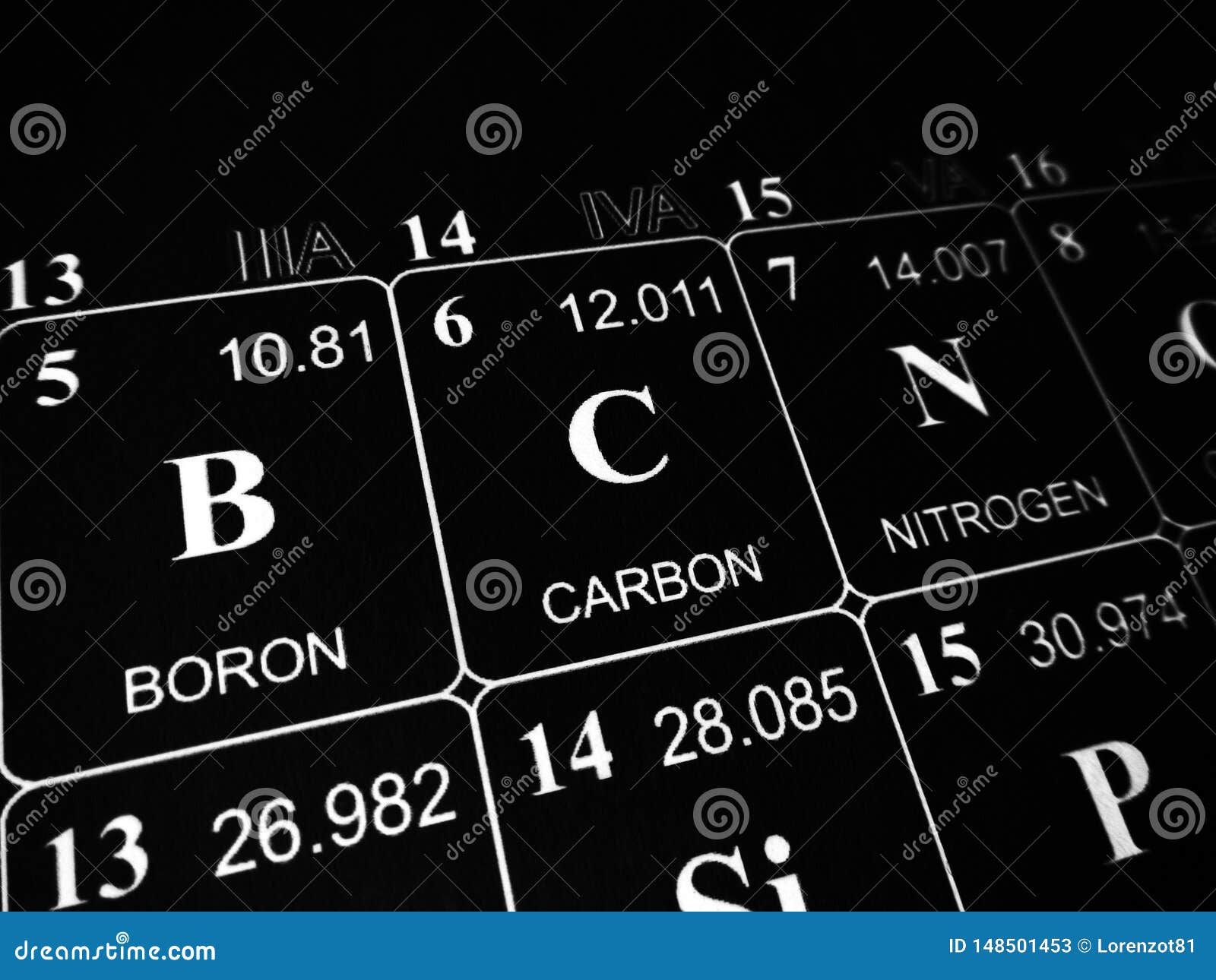
You should note the pattern here compares with the layout of the periodic table. The group is also known as the tetrels (from the Greek word tetra, which means four), stemming from the Roman numeral IV in the group names, or (not coincidentally) from the fact that these elements have four valence electrons (see below). Carbon and silicon can form ionic compounds by gaining four. The usual valency values of some elements are shown in Table 2. The Group 4A elements have four valence electrons in their highest-energy orbitals (ns2np2). This tendency for atoms to behave as though they have a specific number of ‘hooks’ is known as valency, and is sometimes referred to as the combining power of an atom (or a group of atoms). Similarly the chlorine atom also has one ‘hook’, so the prediction for the formula of the compound from carbon and chlorine is CCl 4. The simplest compound of carbon and hydrogen is the gas methane, CH 4: a formula that would be obtained if it were assumed that carbon had four ‘hooks’ and hydrogen had one. If an atom contains a closed shell with valence electrons, it will be chemically alert. Valence electrons, on the other hand, can live in a transition elements inner shell. For main group elements, valence electrons can occur in the outermost shell. Here the number of valence electrons corresponds directly to the number of hooks, the link is not always so simple, as you will see. The periodic table can be used to determine the number of valence electrons in an atom. With four binding sites and a relatively small atomic size, carbon can form chemical bonds with a wide variety of other atoms or. (octet) or empty, giving carbon a usual valence of +4 or -4. See forms of carbon, get interesting facts, and learn about its. Here the number of valence electrons corresponds directly to the number of ‘hooks’. Learn about the element that is atomic number 6 on the periodic table. So, in the valence shell (in this case corresponding to the principal quantum number 2), there are four electrons. The standard atomic mass of carbon is 12.0096 and its symbol is ‘C’. The energy of the orbitals determines the sequence of filling- Lower energy orbitals are always preferred over high energy ones. Carbon is the sixth element in the periodic table and the 1st element in group-14. In a covalent bond, the electrons are shared between the. The electronic configuration of the carbon atom is 1s 2 2s 2 2p 2. The periodic table shows us the sequential filling of the electrons. Since carbon has four valence electrons, it can form 4 covalent bonds. How many valence electrons does carbon have?


 0 kommentar(er)
0 kommentar(er)
